Theme: Bio Markers: Future of Medical Diagnosis and Treatment
Biomarkers 2020
Conference Series LLC Ltd sincerely welcomes every one of the members over the globe to go to the "11th Annual Biomarkers and Clinical Research Congress" during May 29-30, 2020 at London, UK with theme “Bio Markers: Future of Medical Diagnosis and Treatment”.
11th Annual Biomarkers and Clinical Research Congress is anticipating participants from 40 and more countries across the globe and the two days conference will provoke plenary sessions, Keynote speeches, Poster, and Oral Presentations. This program provides two days of robust discussions on recent advancements and new strategies for development of new materials for global requirements.
Details of 11th Annual Biomarkers and Clinical Research Congress 2020 in London.
|
Conference Name |
Place |
Date |
|
Biomarkers 2020 |
London, UK |
May 29-30, 2020 |
Why to attend the Conference?
Biomarkers 2020 Conference brings together the specialists from all the aspects to meet and discuss the future of Clinical Research and Importance of Biomarkers in today’s world to thrive and survive with a better health. Biomarkers assess diseases with distinct applications in analytic epidemiology, biomarkers and clinical research. This session discusses more about clinical research and biomarkers.
This conference discusses technological developments, new discoveries, new approaches, latest trends in research in Biomarkers & Clinical Research. There will be discussions, Interactions, lectures, debates, workshops, plenary talks, keynote addresses, poster sessions etc. This Conference gives you an opportunity to learn more about Biomarkers & Clinical Research.
Target Audience:
Clinical research organizations, Scientists, Biomarker research students, Biomarker researchers, Clinical research scholars, Students, Biomarker associations, Cancer researchers, Oncologists, Radiologists, Pathologists, Pharmacology professionals, Biochemists, Bio-pharmacists, Immunologist, Cancer Associations and Societies, Business Entrepreneurs, Pharmaceutical Companies, and Medical Devices Companies are welcome to participate in this Conference.
Theme: Bio Markers: Future of Medical Diagnosis and Treatment.
Track 1: Clinical Research & Biomarkers
Clinical research biomarkers which are used for clinical purposes are known as clinical biomarkers. Clinical biomarkers provide active and influential way to understanding the scope of many diseases and epidemiology, random clinical trials, screening for drugs or disease diagnosis and prognosis. It is defined as changes in the constituents of cells or body fluids, these clinical biomarkers offer the means for standardized classification of a disease and risk factors that can extend the basic information about the underlying pathogenesis of diseases. The main researches in clinical biomarkers are done in the fields of drug discovery, pharmacogenomics, oncology, and disease diagnostics. Classes of Biomarkers in Clinical trials are segmented into Safety biomarkers & Efficacy biomarkers.
Track 2: Biomarkers in Diagnosis: Challenges and Approaches
Biomarkers and diagnostics play a crucial role in the outcomes and findings in clinical settings to enhance the quality of human health. Translational biomarkers and diagnostics can be applied in both preclinical and clinical setting. It should aim to translate the findings in fundamental research into medical practice and meaningful health outcomes. The characteristics for acceptable translational biomarkers and the various approaches to their selections including the latest trends and developments in translational biomarkers and diagnostics shall be discussed in this session. This session also discusses and reviews methods and requirements for qualification of translational biomarkers.
Track 3: Biomarkers in Drug discovery and development
This biomarker conference throws light on the use of biomarkers in drug development which has emphasised the use of biomarkers as surrogate end points for effectiveness. Biomarkers enhances the understanding of the mechanism of action, enables the assessment of target engagement, facilitates early proof of dose focusing and increases the efficiency of early clinical development with improved quality of decision making, these concepts help in the drug discovery and development process.
Track 4: Biomarkers for Cancer Diagnosis
A cancer biomarker refers to a substance or process that is indicative of the presence of cancer in the body. A biomarker may be a molecule secreted by a tumour or a specific response of the body to presence of cancer. Genetic, epigenetic, proteomic, glycolic, and imaging biomarkers can be used for cancer diagnosis, prognosis, and epidemiology. While some cancer biomarkers can be used to predict how aggressively your cancer will grow, and are therefore useful for assessing your prognosis, the most promising use of biomarkers today is to identify which therapies a patient’s cancer may or may not respond to.
Track 5: Biomarkers of Cardiovascular disorders
Cardiovascular disease (CVD) remains the leading cause of death globally. The identification of traditional risk factors such as age, hypercholesterolemia, hypertension, diabetes mellitus, and smoking has improved primary prevention of CVD. Cardiac markers are measured biomarkers to assess cardiac function. They are often discussed in the context of myocardial infarction, but other conditions can lead to an elevation in the level of the cardiac marker. Cardiac biomarkers are elements that are released into the blood when the heart is damaged or strained. A cardiac marker is used in the identification and risk stratification of patients with chest pain and suspected acute coronary syndrome (ACS). These markers include enzymes, hormones and proteins. Cardiac biomarkers have evolved as essential tools in cardiology in the last 50 years, that is, for primary and secondary prevention, the diagnosis and treatment of acute myocardial infarction (AMI), and the diagnosis and stratification of the risk of heart failure.
Track 6: Biomarkers of Disorders of the Nervous System
In spite of all the advances in neurology, there are serious deficiencies in our understanding of the pathomechanism of several neurological disorders as well as our ability to diagnose and treat these disorders. Biotechnologies are being increasingly applied in neurology to address some of these deficiencies. Novel biomarker identification for neurological disorders will address the current shortcomings in their diagnosis and therapeutics.
Track 7: Biomarkers in Immune-Oncology
The clinical use of biomarkers to survey the impact of safe based malignancy treatments is imperative for a few reasons. To start with, invulnerable based medicines, for example, immunizations, are frequently intended to inspire a particular reaction so the estimation of that reaction could be a marker of item (e.g., antibody) strength. Also, as safe based treatments are tried before in the restorative pathway (e.g., in the adjuvant setting), biomarkers of reaction turn out to be progressively imperative as potential endpoints of clinical preliminaries. At long last, clinically qualified biomarkers are required with the goal that new immunotherapies can be quickly and effectively tried and meant clinical practice.
Track 8: Biomarkers for Early Diagnosis and Prognosis
Biomarkers focusing on two major areas of investigation: the early detection and prognosis. Early diagnosis and Prognostic Research encompasses the research addressing studies on the evaluation of medical tests, markers, prediction models and decision tools. Early diagnosis of cancer generally increases the chances for successful treatment by focusing on detecting symptomatic patients as early as possible. Delays in accessing cancer care are common with late-stage presentation, particularly in lower resource settings and vulnerable populations. The consequences of delayed or inaccessible cancer care are lower likelihood of survival, greater morbidity of treatment and higher costs of care, resulting in avoidable deaths and disability from cancer. Early diagnosis improves cancer outcomes by providing care at the earliest possible stage and is therefore an important public health strategy in all settings.
Track 9: Biomarkers for non-cancerous diseases
Biomarkers plan an important role in various neurological and cardiovascular diseases. The diseases which are incurable from ages are being offered a possible treatment and easy diagnosis with the help of advancements in biomarkers through different diagnostics and imaging technologies. Biomarkers are used in many other non-cancerous diseases.
Track 10: Biomarkers in Disease Treatment
Identification and validation of discovered gene or protein-based, network or dynamic network biomarkers with human diseases, patient phenotypes, or clinical applications, and accelerate the development of human disease-specific biomarkers for the early diagnosis, monitoring, evaluation, and prediction of diseases. Conditions including cancers, cardiovascular and metabolic diseases will be the focus of study. This section will promote the innovation and development of disease-specific biomarkers by integrating multidisciplinary aspects of science.
Track 11: Biomarkers based on epidemiological investigations
Biomarkers have been classified based on the epidemiological investigations exposure to disease & biomarkers of disease which are also used in the investigation of the natural history and prognosis of a disease. In addition to the factors between exposure and disease, biomarkers have the potential to identify the earliest events in the natural history, reducing the rate of misclassification of both disease and exposure, leads to increase potential mechanisms related to the disease pathogenesis, accounting for some of the variability and effect modification of risk prediction. These epidemiological investigations are one of the reasons behind biomarkers to improve validity while reducing bias in the measurement of exposures (or risk factors) for neurological disease.
Track 12: Biomarkers & Next-Generation Sequencing
The biomarker congress focuses on the biomarker validation which is an open-ended process with open-ended evidentiary standards, where every potential application of the biomarker needs to be supported by independent studies and datasets. One aspect that has created a revolution in the concept of biomarker research and development is the Next Generation Sequencing which is versatile analysis tool for medical and biological research. Next Generation Sequencing technology refers to one high-throughput DNA sequencing method. In a single experiment, it can determine the sequence of the target gene or full genome with a total size of larger than millions of base pairs. Sequencing thousands of genes or even genomes in one experiment is consequently made possible using this NGS technology.
Track 13: Genomic Biomarkers in Clinical Development
Genomics and Clinical development promise the development of biomarkers to a state to predict the risk of individual disease that allows early detection of the disease and improves diagnostic classification to better inform individualized treatment. Biomarkers are biological measurements that can be used to predict the risk of diseases, to allow early detection of diseases, to improve the selection of treatment and to monitor the outcome of therapeutic interventions. The main objective of the Human Genome Project was the identification and development of such biomarkers for "personalized, preventive and predictive medicine”. This clinical conference focuses on biomarkers incorporating in the gene level into earlier stages of the clinical trials which help to separate the patients and help in the development of the biomarker and in case of clinical development this incorporation serves in multiple ways from guiding dose selection to selecting the mode of action to providing the strategy to know about to whom the a particular biomarker can be used.
Track 14: Biomarkers: Validation & Verification
Biomarker discovery requires high confidence identification of biomarker candidates with simultaneous quantitation information to indicate which proteins are changing to a statistically relevant degree in response to disease. Because of normal clinical or biological variability, candidate biomarkers identified in the discovery stage need to be validated across a large number of samples. The challenge is to develop a fast, targeted analysis method capable of analysing as many identified candidates as possible in minimally hundreds and potentially even thousands of samples.
Track 15: Imaging Technologies and Diagnostic tests of Biomarkers
In clinical trials using imaging, biomarkers and response criteria are used to assess the tumor evolution with therapy. Imaging biomarkers are often root contributors to a clinical trial’s endpoints, but they are not the same. Diagnostic tests and the biological biomarker (biomarkers) they measure are often classified based on the circumstances under which they are used. Biomarkers provide information about a patient at virtually every stage of care. They can help doctors evaluate the likelihood that a patient will develop a disease, diagnose a disorder, evaluate the severity of a disorder and/or its likely progression, determine optimal treatment strategies and monitor response to treatment.
Track 16: Clinical and Biomedical Engineering
Biomedical Engineering is the science of application of engineering principles to the fields of biology and health care. Bioengineers work with doctors, therapists and researchers to implement systems, equipment and devices in order to solve clinical problems which focus on the advances that improve human health and health care at all levels. Clinical engineering is a special field within Biomedical engineering responsible primarily for applying and implementing medical technology to optimise healthcare delivery.
Track 17: Entrepreneur Investments Meet
A key fixing in fruitful business is self-learning. Biomarkers-2020 plans to unite all current and growing bio-business people to share encounters and present new developments and difficulties inside the malignant growth network. Every year, over a million organizations work on the planet with about 5–10 of them being named high innovation organizations. Transforming thoughts into business adventures is precarious and the open door acknowledgment step is basic in new pursuit manifestations. This gestalt in the business visionary's view of the connection between the creation and last item is refined into a plan of action that depicts how the endeavour will profit or give a fitting come back to the potential financial specialists. Disease science is perplexing and quickly changing and requires specific information to comprehend the estimation of the advancement and its aggressive position in the business. This multi -day network wide gathering will be a very intelligent discussion which will get specialists zones extending from Biomarkers to flagging pathways to novel helpful ways to deal with the logical centre point.
Overview on biomarkers market:
The global biomarkers market has increased demand in recent years due to the rapid growth of population, rapid growth in IT industry which leads to increasing efforts towards the drug discovery. The entry of new entrepuners is expected to further boost the growth of the market. The advancements and innovations of biomarkers in medical arena such as research and cancer treatment will propel the future growth of the global biomarkers market. Biomarkers are broadly used to detect diseases such as immunological disorders, neurological disorders, cancer, cardiovascular disorders, and others. In the arena of oncology, recently three biological markers have been added to conventional breast cancer risk models to identify women at a higher risk of breast cancer
Global Vocal Biomarkers Market:
Sound vibrations are used as part of therapeutic healing for various mental health conditions such as anxiety and depression. Recent research studies, however, have greatly widen its applications into diagnosis of various diseases. On successful completion of clinical trials, HIPAA compliant vocal biomarker system would serve as much more alternative methods to current diagnostic techniques such as MRI, X-Ray, and CT scan. Research is also ongoing for identifying vocal biomarkers in diagnosis of Parkinson’s disease, traumatic brain injury, cognitive impairment, and respiratory disorders, which would open up a highly profitable avenue for growth for players in this industry. Since last 22 years companies dealing with vocal biomarkers is on researching by sampling data to provide accurate results and it has collected over 2.5 million voice samples in over 40 different languages.
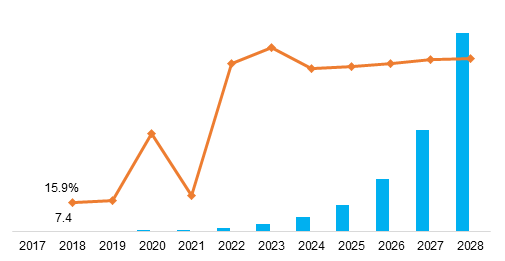
Global Biomarkers Market is expected to USD 78.9 billion by 2024, from USD 31.4 billion in 2016 growing at a CAGR of 12.2% during the forecast period of 2017 to 2024. The new market report contains data for historic years 2014 & 2015, the base year of calculation is 2016 and the forecast period is 2017 to 2024.
Regional analysis includes
- North America (U.S., Canada)
- Latin America (Mexico, Brazil)
- Western Europe (Germany, Italy, U.K, Spain, France, Rest of Western Europe)
- Eastern Europe (Russia)
- Asia Pacific (China, India, ASEAN, Australia & New Zealand)
- Japan
- Middle East and Africa (GCC, S. Africa)
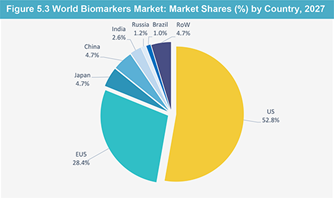
Summary (Overall Market of Biomarkers & Clinical Research with Statistics):
The global biomarkers market is expected to reach $45.55 Billion by 2020 from $24.10 Billion in 2015, at a CAGR of 13.58% between 2015 and 2020. Increasing healthcare expenditure & R&D spending and the increasing utility of biomarkers for diagnostics are expected to drive the market. Market growth will also be aided by the low cost of clinical trials in developing countries and new initiatives undertaken for biomarker research. On the other hand, the need for high capital investment, low benefit-cost ratio, poorly suited regulatory & reimbursement systems, and the high cost of tests and sample collection & storage are the major factors restraining the growth of this market.
The applications included in this report are diagnostics development, drug discovery & development, personalized medicine, disease risk assessment and other applications. The disease indication segments included in this report are cancer, cardiovascular disorders, neurological disorders, immunological disorders, and other diseases.
Major players in this market include QIAGEN N.V. (Netherlands), PerkinElmer, Inc. (U.S.), Merck & Co, Inc. (U.S.), Bio-Rad Laboratories (U.S.), Enzo Biochem (U.S.), EKF Diagnostics Holdings plc (U.S.), Meso Scale Diagnostics, LLC (U.S.), Singulex, Inc. (U.S.), BioSims Technologies (France), Cisbio Bioassays (France), and Signosis, Inc. (U.S.).
The success of the Biomarkers & Clinical Research Conference has given us the prospect to bring the congregation one more time. Conference Series LLC Ltd hosted the “10th World Congress on Biomarkers & Clinical Research” during October 03-04, 2018 in Los Angeles, USA.
The conference was focused on Biomarkers & Clinical Research studies with the theme ‘Latest advancements in the field of Biomarkers & Clinical Research”. The conference was embarked with an opening ceremony followed by Keynote sessions and followed by series of lectures delivered by Honourable Guests and members of the Keynote forum. The adepts who promulgated the theme with their exquisite talk were.
Conference Series LLC Ltd offers its heartfelt appreciation to Organizing Committee Members, dexterous of field, various outside experts, company representatives and is obliged to other eminent personalities who interlaced with Conference Series LLC Ltd and supported the conference in every aspect, without which the conference would not have been possible.
Conference Series LLC Ltd would like to announce the commencement of the “11th Annual Biomarkers and Clinical Research Congress” to be held during May 29-30, 2020, London, UK. We welcome all the eminent researchers, students and delegate participants to take part in this upcoming conference to witness invaluable scientific discussions and contribute to the future innovations in the field of Biomarkers & Clinical Research.
For more details: https://biomarkers.conferenceseries.com/
Let us meet again @ Biomarkers 2020
Conference Highlights
- Clinical Research & Biomarkers
- Biomarkers Diagnosis in Drug Formulation :
- Biomarkers for Cancer Diagnosis
- Biomarkers for Early Diagnosis and Prognosis
- Biomarkers in Disease Treatment
- Biomarkers & Next-Generation Sequencing
- Genomic Biomarkers in Clinical Development
- Biomarkers: Validation & Verification
- Biomarkers for Non-Cancerous Diseases
- Entrepreneur Investments Meet
- Biomarkers in Drug discovery and development
- Biomarkers of Cardiovascular disorders
- Biomarkers of Disorders of the Nervous System
- Biomarkers in Immune-Oncology
- Biomarkers based on epidemiological investigations
- Imaging Technologies and Diagnostic tests of Biomarkers
- Clinical and Biomedical Engineering
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | May 29-30, 2020 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Cancer Science & Therapy
- Journal of Carcinogenesis & Mutagenesis
- Journal of Oncology Research and Treatment
Abstracts will be provided with Digital Object Identifier by







